GIN Home
Ichneumonid Morphology
Subfamily Key
Lists of World Genera
Acaenitinae
Brachycyrtinae
Collyriinae
Lycorininae
Ophioninae
Poemeniinae
Rhyssinae
Stilbopinae
Xoridinae
By Ian D. Gauld & David B. Wahl
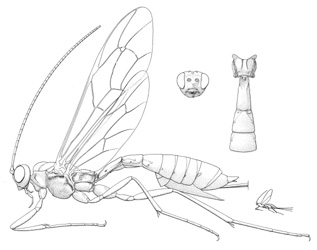
Coleocentrus exitator |
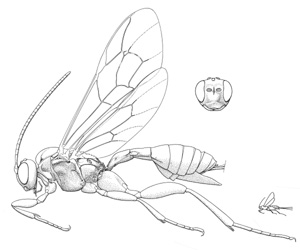
Mesoclistus rufipes |
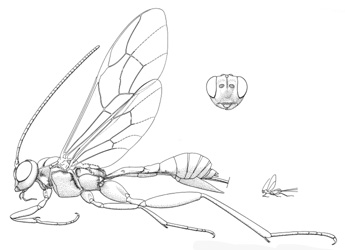
Spilopteron franclemonti |
The subfamily Acaenitinae is notable for the female hypopygium, which is large and triangular, folded on the midline, and reaching or surpassing the metasomal apex. Many are brightly patterned; combined with the long ovipositor found in all species, acaenitines can be quite striking. Despite these features, most acaenitines are rather infrequently collected insects. As one would expect from their morphology, acaenitines are parasitoids of insects mining in plant tissue, but much remains to be discovered about their biology and host relationships.
Classification and diversity
The Acaenitinae worldwide includes about 250 species placed in 25 genera (Yu & Horstmann, 1997). Wahl and Gauld (1998) demonstrated that this subfamily belongs within the Pimpliformes and is the sister group to a clade of Diptera-parasitizing subfamilies (Cylloceriinae, Diplazontinae and Orthocentrinae). Some authors (Townes, 1971; Constantineanu & Pisica, 1977) have recognized two tribes, the Acaenitini and the Coleocentrini. Wahl and Gauld (1998) demonstrated that the latter was a paraphyletic group that included six genera of basal acaenitines and thus no longer warrants recognition. Many of these basal genera principally occur in the southern part of the Eastern Palearctic region (such as Hallocinetus in Romania and Armenia, and Eremocinetus in Tadzhikistan), and all six genera are restricted to the Holarctic region. Only two, (Coleocentrus and Mesoclistus, are represented in North America, the former by eight species and the latter by a single species present in Alaska.
The remaining 18 genera clearly form a monophyletic group and have an Old World center of distribution. They are well-represented in the Eastern Palaearctic and by a small number of rather species-rich radiations in the wet forests of the Afrotropical region and Southeast Asia, as well as a small group of Yezoceryx species in tropical wet Australia (Gauld, 1984). Three genera are represented in North America: Arotes, Spilopteron and Yezoceryx. Most of these Nearctic species are restricted to the cooler northeastern states, with species-richness declining markedly southwards. The group is more or less absent from tropical America, with only a single species, Arotes pammae, found south of the Isthmus of Tehuantepec, (and this species ranges only as far south as Costa Rica (Gauld, 1991)). This pattern of distribution suggests a Palaearctic origin for the Acaenitinae, with subsequent radiation into Africa and southeastern Asia and a trans-Bering migration into the New World.
The general biology of Acaenitinae
Rather little is known of the biology of acaenitines, but the host-lists of most catalogues and taxonomic revisions consist of wood-boring insects: Coleoptera, Hymenoptera (Siricidae/Xiphydriidae) and Lepidoptera (Sesiidae) (Bajári, 1960; Aubert, 1969; Constantineanu & Pisica, 1977). Nearctic acaenitines are recorded as parasitoids of larvae of the beetle families Cerambycidae, Melandryiidae and Mordellidae (Townes & Townes, 1960; Carlson, 1979).
It has been assumed by some authors that acaenitines are ectoparasitic idiobionts like most other parasitoids of wood-boring Holometabola (Aubert, 1969) but this is not necessarily so. While Coleocentrus has been observed to parasitize various holometabolous insects (siricids, sesiids, and xylophagous coleopterans) living in wood (Baumann, 1927, 1933; Townes & Townes, 1960), little else is known of their biological habits. Some of the more derived genera are koinobiont endoparasitoids (Shaw & Wahl, 1989; Zwakhals, 1989). These authors observed that Acaenitus dubitator develops as a koinobiont endoparasitoid of an endophytic curculionid. The female wasp oviposits into first and second instar host larvae, and parasitization appeared to retard host development. Three ichneumonid larval instars were discerned and the final instar apparently killed its host about the time unparasitized weevil larvae were pupating. The acaenitine larva then spun a tough parchment-like cylindrical cocoon within the hosts pupation chamber (Shaw & Wahl, 1989).
Larval morphology suggests that Coleocentrus is an endoparasitoid: the position of the spiracular closing apparatus with respect to the atrium, reduced skin setae, lack of antennal papillae, and the restriction of mandibular base sclerotization to the bases periphery (Wahl, 1986). This implies that acaenitines are all endoparasitoids, although there is still a question of idiobiosis vs. koinobiosis as part of the subfamilys groundplan. Wahl & Gauld (1998: 284-286) discuss the various possibilities in some detail.
Diagnosis
Small to large ichneumonids, fore wing length 5 to 20 mm. Labrum usually exposed, semicircular or elliptic; hidden and with notched apex in Coleocentrus. Clypeus transverse and basally flat, with weak to strong transverse ridge that varies in position from midpoint to apical 0.8, remaining apical portion of clypeus thinner (when transverse ridge strong, position is usually near apex which thus appears thickened); epistomal suture present (albeit often weakly) or absent; apex strongly concave or truncate/weakly concave, with prominent median tubercle in Coleocentrus. Malar space with subocular groove present or absent. Mandible short, apex usually with two teeth (one tooth in Paracollyria). Supra-antennal area sometimes with crest between antennal sockets. Antenna with flagellum filiform; males without tyloids. Ventral posterior corner of propleuron without strongly produced lobe. Pronotum with epomia present dorsally, rarely extending ventrally on anterior pronotal margin. Notauli usually strongly impressed and extending posteriorly towards scuto-scutellar groove. Epicnemial carina present or absent. Sternaulus absent. Posterior transverse carina of mesothoracic venter absent or present only as central vestige. Propodeum long, with carinae varying from complete to absent. Claws of fore and middle tarsi simple or with appressed acute tooth near apex, or in Hallocinetus with large basal lobe; hind tarsal claws simple or with appressed acute tooth. Fore tibia with dorsal apical tooth absent. Fore wing with areolet present (petiolate triangular) or absent; vein 2m-cu with two short widely interspaced bullae. Hind wing with vein 1/Rs = vein rs-m; veins 1-Rs, 1-M and 2-Cu tubular to nebulous. Metasomal insertion on propodeum between hind coxae. Metasoma can be cylindrical or dorsoventrally depressed, but usually with apical 0.3-0.5 moderately laterally compressed. Metasomal segment 1 with T1 narrow anteriorly, posteriorly evenly broadened, with spiracles in front of midpoint; glymmae present or absent; T1 and S1 free or fused; sclerotized anterior section of S1 short and not reaching beyond midpoint. Thyridium present, ovoid to transverse, usually immediately adjacent to base of T2. T2-4 usually smooth; T2-3 with basolateral grooves in Coleocentrus. Hypopygium of female large, triangular in profile, and folded on midline, its apex usually reaching or surpassing metasomal apex (hypopygial apex notched and not reaching metasomal apex in Eremocinetus, Leptacoenites, and Procinetus). Ovipositor projecting beyond apex of metasoma by at least length of hind tibia and frequently longer; upper valve without dorsal subapical notch, apex of lower valve usually with teeth (which may be weak).
The genera of Acaenitinae present in the Nearctic
- Arotes Gravenhorst, 1829. Holarctic, Neotropic: four described Nearctic species. (HOD)
- Coleocentrus Gravenhorst, 1829. Holarctic: eight described Nearctic species. (HOD)
- Mesoclistus Förster, 1869. Holarctic: one described species in Nearctic (cushmani Townes). (HOD)
- Spilopteron Townes, 1960. Holarctic, Oriental: four described Nearctic species. (HOD)
- Yezoceryx Uchida, 1928. Australian, Holarctic, Oriental: one described species in Nearctic (rupinsulensis (Cresson)). (HOD)
Identification and resources
While female acaenitines are easily identified by their large triangular hypopygia, recognizing males is much harder for the non-specialist. They will probably be initially placed in the Pimplinae. Arotes and Spilopteron males can be segregated by: 1) the fore and middle tarsal claws have small subapical teeth, and 2) the clypeus has a preapical transverse ridge that makes its apex appear to be thickened. Males of Coleocentrus have: 1) a median tooth on the clypeal apex, and 2) vein 1/Cu of the hind wing 0.1x as long as vein cu-a.
Key to the genera of Nearctic Acaenitinae
· Downloadable pdf file of the key is available here
· Downloadable pdf file of the key figures is available here
Hymenoptera Online Database (HOD)
Keys to the Nearctic species are given by Townes & Townes (1960).